|
|
 |
|
|
IntellaTurn's Weekly Scoop
By Erin at IntellaTurn ● Feb 26, 2026
The latest: Drug approvals | Plausible mechanism pathway | Around the industry | Gene therapy access issues
|
|

iStock / Ton Photograph
✅ Sanofi and Regeneron’s DUPIXENT: First treatment for allergic fungal rhinosinusitis (AFRS)
-
The FDA granted approval to Dupixent (dupilumab) for treating patients aged 6 years and up with allergic fungal rhinosinusitis and a history of sino-nasal surgery, marking the first treatment approved for this indication, according to a press release.
-
The release noted that the FDA’s decision on Dupixent was, in part, based on findings from the LIBERTY-AFRS-AIMS trial.
“Before Dupixent, people living with allergic fungal rhinosinusitis had to rely on treatments that left them potentially vulnerable to regrowth of nasal polyps and thick mucus that could rob them of their sense of smell,” Alyssa Johnsen, MD, PhD, global therapeutic area head, immunology development at Sanofi, said in the release. (Healio)
✅ Immedica’s LOARGYS: First treatment for hyperargininemia in Arginase 1 deficiency (ARG1-D)
-
Swedish biotech Immedica won an accelerated approval from the FDA, resurrecting the first treatment to address persistently elevated levels of plasma arginine, which is the primary driver of the rare disease ARG1-D.
-
The approval of Loargys, an arginine-specific enzyme, follows a complete response letter in August and Immedica’s acquisition of the compound in 2022 after the bankruptcy of Aeglea BioTherapeutics.
-
Aeglea in 2022 received a refuse-to-file letter from the FDA, which sought more “evidence showing that plasma arginine and metabolite reduction predicts clinical benefit in patients with ARG1-D.”
-
Immedica will win a priority review voucher as part of the approval, which could provide the KKR-backed biotech with $100 million to $200 million when it’s sold. (Endpoints)
✅ AstraZeneca’s CALQUENCE combination: First all-oral, fixed-duration regimen for 1st line CLL or SLL
-
AstraZeneca secured a key regulatory win in its effort to reclaim the lead in the BTK inhibitor market, with the FDA approving its combination of Calquence plus Venclexta as the first all-oral, fixed-duration regimen for first-line chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL).
-
While patients with newly diagnosed CLL typically take a BTK drug like Calquence indefinitely until disease progression, the new approval offers a better quality-of-life alternative by limiting treatment to 14 months.
“The continuous regimens frequently used to treat chronic lymphocytic leukemia often come with side effects that may become burdensome to patients over time,” Jennifer Brown, MD, PhD, from the Dana-Farber Cancer Institute, said in a statement, adding that the Calquence combo gives doctors greater flexibility to tailor treatment plans. (Fierce Pharma)
✅ Vanta’s BYSANTI: Novel atypical antipsychotic for acute bipolar I and schizophrenia
-
After concluding last year on solid footing with FDA approval of its motion sickness drug Nereus (tradipitant), Vanda announced that the regulator has now authorized the atypical oral antipsychotic Bysanti (milsaperidone) for the acute treatment of manic or mixed episodes associated with bipolar I disorder and for schizophrenia in adults.
-
Vanda explained that Bysanti builds on its well-established therapy Fanapt (iloperidone), approved earlier for the same indications.
-
The therapy, which is likely to launch in the third quarter, quickly interconverts to Fanapt in vivo, producing dual active molecules that antagonise dopamine D2, serotonin 5-HT2A and alpha1-adrenergic receptors.
-
The company noted that in clinical studies Bysanti demonstrated bioequivalence to Fanapt, with its NDA filing last year supported by the latter’s existing clinical data, including two acute schizophrenia studies, one bipolar I study, and a schizophrenia relapse-prevention trial. (FirstWord Pharma)
|
|
FDA smooths reviews of rare disease treatments

iStock / Grandbrothers
What’s new: The FDA laid out a new “plausible mechanism” framework aimed at spurring novel individualized treatments targeting rare diseases.
Why it matters: Officials said the draft guidance would make it easier for industry to develop more types of promising new treatments for rare diseases, including with CRISPR gene-editing technology.
Driving the news: The agency is establishing a way to evaluate rare disease treatments that can’t be put through traditional clinical trials because the patient populations are too small.
The big picture: The move was inspired by “Baby KJ,” an infant born with a rare metabolic disease who became the first person to be successfully treated with a personalized CRISPR therapy last year at Children's Hospital of Philadelphia.
Go deeper: Axios
|
|

Axios Media
💵 Fortress sells FDA voucher for $205M after ZYCUBO approval
-
Only a month after Jazz Pharmaceuticals said it had signed a deal to sell an FDA priority review voucher (PRV) for $200 million, a new PRV transaction involving Fortress Biotech and an unnamed buyer shows that the trend of rising voucher prices is still going strong.
-
On February 23, Fortress said its subsidiary, Cyprium Therapeutics, has entered into an agreement to sell a recently received rare pediatric disease priority review voucher for $205 million.
-
Cyprium got the PRV as part of the FDA’s recent approval of injected copper replacement therapy Zycubo as the first treatment approved in the US for the rare neurodegenerative disorder Menkes disease.
-
While another company, Sentynl Therapeutics, is handling development and commercialization of Zycubo under a 2023 agreement, the deal called for Sentynl to transfer the PRV back to Fortress/Cyprium after the approval. (Fierce Pharma)
🫁 GSK picks up pulmonary hypertension drug with 35Pharma acquisition
-
Building up its base of medicines for lung diseases, GSK said Wednesday it was buying the privately held firm 35Pharma for $950 million in cash.
-
The centerpiece of the deal is an experimental drug called HS235 that is set to start trials in pulmonary arterial hypertension, a form of high blood pressure in the lungs.
-
The disease has increasingly become a target for drugmakers in recent years. Merck’s Winrevair won approval for PAH in 2024, and reached $1.4 billion in sales last year.
-
35Pharma, based in Canada, has completed Phase 1 safety trials of HS235. In addition to the PAH trial, GSK plans to test it in a related disease called pulmonary hypertension due to heart failure with preserved ejection fraction. (STAT)
🏭 Roche seeks antibiotic partner as rising manufacturing costs drive supply rethink
-
Roche has started looking for a partner to handle production and supply of Rocephin after concluding it is becoming “increasingly unsustainable” to make the antibiotic in Switzerland.
-
Rocephin is Roche’s brand name for ceftriaxone, a long-acting, broad spectrum cephalosporin antibiotic. Since ceftriaxone was approved in the 1980s, physicians have used the injectable antibiotic to treat conditions such as sepsis and meningitis in children and adults. The antibiotic is on the World Health Organization’s list of essential medicines.
-
Roche serves the market from its production site in Kaiseraugst, Switzerland. Yet after manufacturing the antibiotic for 40 years, Roche is now seeking a production partner. (BioSpace)
|
|
Interesting read: Access to gene therapy depends on where you live
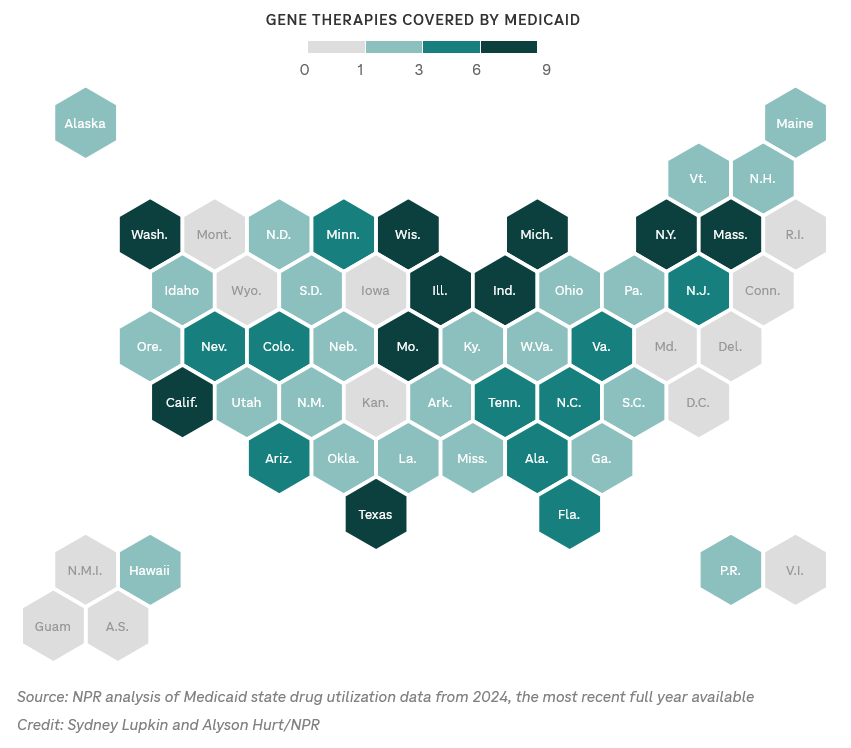
Without a central reporting system, it’s hard to know how many people have received cutting edge gene therapies. But an NPR analysis of state Medicaid data offers a snapshot. It shows location matters — and that there are gene therapy deserts.
About gene therapies: Usually one-time treatments, they break into cells and modify the disease-causing genes.
“These therapies are incredible,” says Dr. Will Shrank, a former Harvard Medical School professor who’s also worked for insurance companies such as Humana and CVS Health.
“They can absolutely massively impact the lives of patients with terrible conditions that, when I was in medical school, none of us could imagine were curable or addressable.”
Yes, but: They can cost a few million dollars for just one patient.
“The fragmented way that we pay for and deliver care in this country is perfectly misaligned with equitably delivering those therapies to patients who can benefit,” says Shrank.
Lack of central reporting systems: The exact number of patients who’ve received these kinds of treatments is hard to pin down because there's no central reporting system, says Sarah Kikkert, a spokesperson for the American Society of Gene and Cell Therapy.
Location matters: An NPR analysis of Medicaid data offers a snapshot that shows location matters. The data is from 2024, the most recent full year available, and while it doesn’t include the total number of patients who benefited, it shows the number of different kinds of therapies each state program paid for that year.
-
State Medicaid programs in Nebraska, North and South Dakota and Oklahoma paid for relatively few gene therapies, for example.
-
Meanwhile, states including California, Indiana, Massachusetts and Texas paid for more.
Further context: NPR showed its analysis to Ameet Sarpatwari, a professor of population medicine at Harvard who studies drug policy. He says states that paid for more gene therapies tended to have more academic medical centers.
-
In theory, even if someone’s state doesn’t have a hospital that offers gene therapy, the state Medicaid program is supposed to pay for that person to get it in another state, Sarpatwari says.
-
But NPR’s analysis shows that isn’t happening, he says, “and that’s troubling.” (NPR)
|
|
✨ Thanks for reading! ✨
🌐 About us: IntellaTurn, LLC delivers business-critical and timely information to biopharmaceutical companies and start-ups in the life sciences industry.
📧 Questions? If you liked this, but need more tailored business insights, get in touch about our curated daily, weekly, and bi-weekly newsletters.
|
|
|
|
|
|
|
|






